Understanding Metal Corrosion in Boats
Metal corrosion in boats is envitable. From the moment your boat enters the water it starts to rot, corrode or rust. This process will continue relentlessly and your boats hull, or any metal components under the waterline, will never be in as good a condition again.
What you can do as a responsible boat owner is monitor the condition of your Hull at certain intervals and take some remedial measures at the appropriate time to prolong your vessels serviceable life.
why does metal corrode in water?
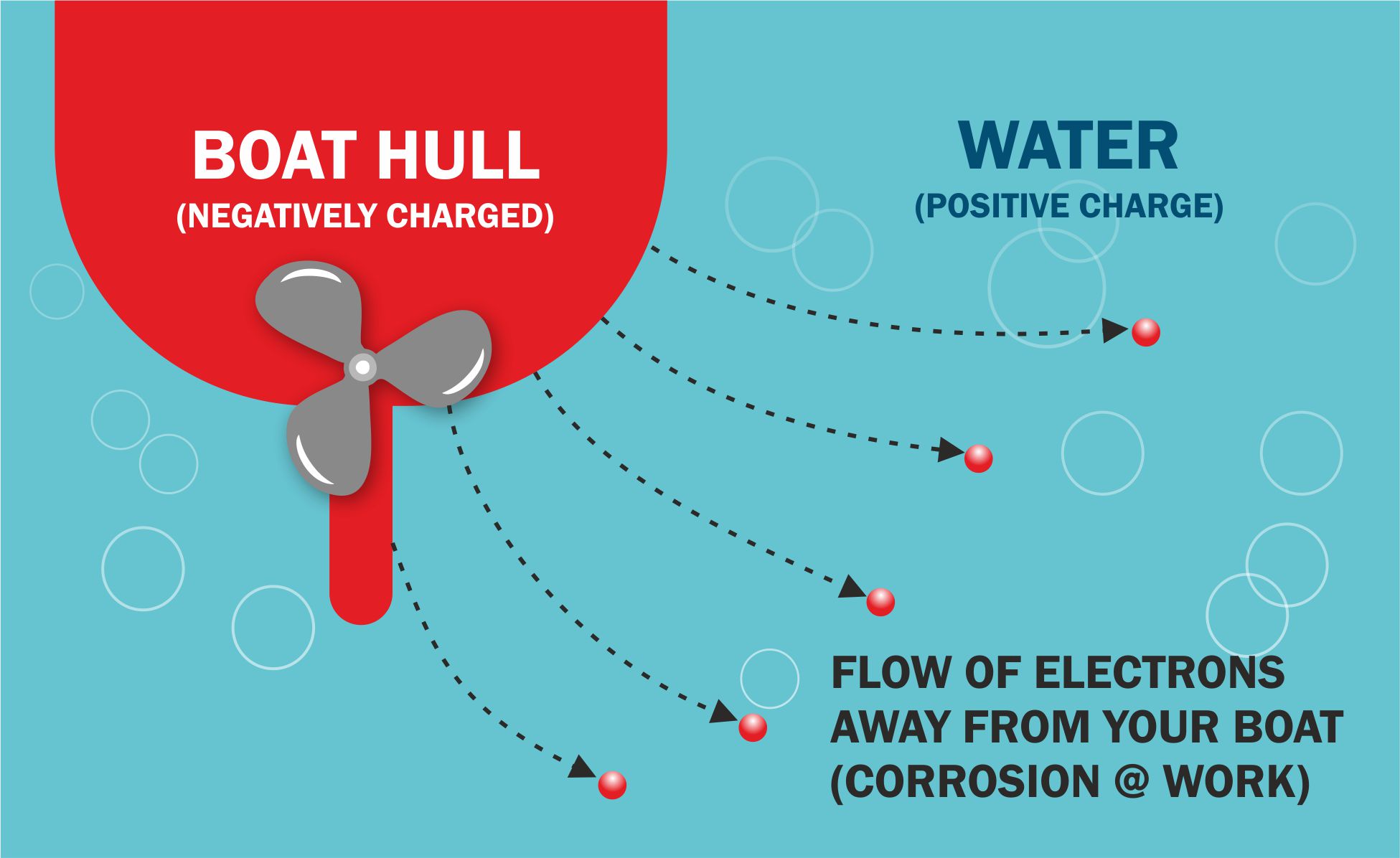
Metals are strong. The metal atoms are arranged in rigid structures and they are surrounded by electrons which are relatively fluid. This allows metal to conduct electricity which is fundamentally the flow of electrons.
Your Hull and any other Metals in contact with the water therefore has a ready supply of electrons which are negatively charged. Unfortunately the water that your Craft is floating in has a positive charge and is permanently looking for sources of electrons to become more stable. Any metal in water is a perfect target.
Not all water is the same. If the water is slightly acidic, more Salty or has more Oxygen (fast flowing or white water) it will be more positive and able to steal electrons more readily from your Boat.
Removal of electrons is a process called Oxidation other words for this include corrosion or rotting. See diagram above.
Fortunately, you can measure both the availability of electrons in your Hull and the corrosive potential of the water and take appropriate action!!
UNDERSTANDING HULL POTENTIAL
To fully understand what is happening with your hull and/or anodes you have to measure the potential of your hull and other metal parts that are submerged.
Measuring Hull Potential is very simple and quick.
Using our Boat Meter Kit simply connect the Hull to the positive terminal and place the electrode in the water near the hull. See here
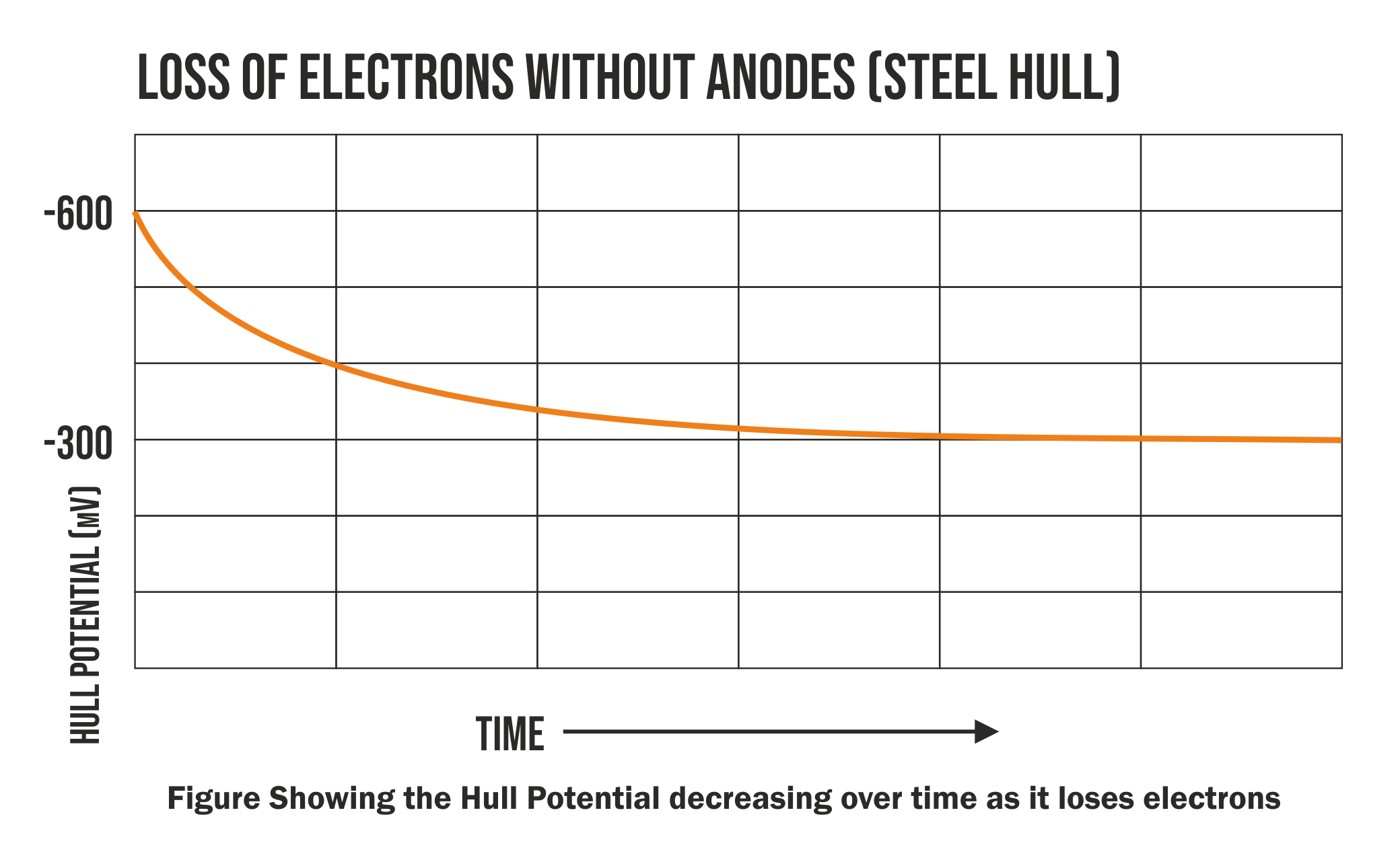
The readings for new boats will be as follows e.g.
Aluminium Hulls -900mv and with Steel Hulls -600mV
The results are negative as electrons are negatively charged. The more negative the result is the more electrons you have locked up in the Hull. This is a good thing.
As the water steals electrons and corrosion occurs this result will become less negative.
Allow us to demonstrate with a graph.